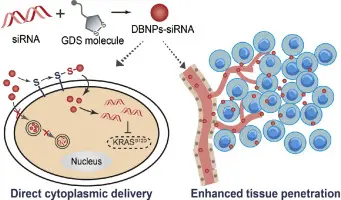
RNA interference (RNAi), a revolution in biology, represents a completely new approach to "silence" disease relevant genes and has the potential to become a whole new class of therapeutic products.
RNAi is a naturally occurring mechanism to destroy messenger RNA (mRNA) and thereby reduce expression of proteins involved in pathological processes. The process starts with the cleavage of input dsRNA into 19-23 base pair (bp) short interfering RNAs (siRNAs) by the enzyme Dicer in a processive and ATP-dependent manner. Noteworthy, based on the mode of action RNAi can, in principle, be used against any of the 30 - 40,000 human genes. In contrast to other classes of therapeutics such as proteins, antibodies and small molecules, RNAi requires neither "drugable" domains on the molecular target (e.g. kinase domains) nor specific locations at the cell membrane or extra-cellular.
Furthermore, siRNAs constitutes a well defined chemical entity. Even different siRNAs molecules (sequences) against the same or a different target gene will have similar ADME and toxicology/safety profiles in animals and humans. This implies that once the first siRNAs molecules have been approved for marketing by the regulatory authorities, the development and regulatory process for subsequent siRNAs molecules will be facilitated and more cost efficient. This is not the case for small molecules or antibodies, where each new chemical entity (NCE) commonly has a different composition and synthesis protocol.
How RNAi is Applied as a Therapeutic
RNAi-based therapeutics use synthetic siRNAs (or shRNAs — short hairpin RNAs) designed to target the mRNA of disease-associated genes. By delivering these molecules into cells, researchers can reduce or completely silence the production of harmful proteins.
RNAi therapeutics hold promise for a wide range of conditions
Genetic disorders caused by overactive or mutant genes.
Cancers, where silencing oncogenes can slow or stop tumor growth.

Viral infections, by targeting viral RNA to prevent replication.

Metabolic and neurodegenerative diseases, where abnormal protein accumulation drives disease progression.
Key Challenges: Delivery and Specificity
Efficient Delivery
siRNAs are large, negatively charged molecules that do not easily cross cell membranes. Developing delivery systems that protect siRNAs from degradation in the bloodstream and guide them into the correct cells or tissues is critical. Lipid nanoparticles (LNPs), viral vectors, and conjugation to targeting ligands are some of the delivery strategies being developed.
Off-Target Effects
siRNAs must be designed to avoid unintentional binding to similar but unintended mRNA sequences. Advanced bioinformatics and chemical modifications help improve specificity and reduce the risk of off-target silencing.
Stability and Immunogenicity
Naked siRNAs are quickly degraded by enzymes in the body. Chemical modifications, such as 2’-O-methyl or locked nucleic acid (LNA) modifications, can increase stability and reduce immune responses.