In the fast-evolving world of biomedical research, target validation is a pivotal step in developing effective therapies. Whether it's a specific gene, a protein, or a biological pathway, researchers must first confirm that the target plays a critical role in the disease process. Without this validation, the likelihood of success decreases, leading to failed trials, wasted resources, and delays in patient care. To help ensure the success of new treatments, scientists today have access to a variety of advanced tools that significantly enhance the target validation process.


Understanding Target Validation in Therapeutic Development
Target validation is the process of confirming that a specific biological target — a gene, protein, or pathway — is directly involved in a disease and that modulating this target can have therapeutic effects. This process is critical because it helps identify the best drug candidates for a particular disease.
A strong validation ensures that researchers are focusing their efforts on biologically relevant targets that will yield therapeutic benefits. Without validation, researchers may spend time and money developing drugs for targets that aren’t effective or essential for treating the disease, which can result in costly clinical failures.
The Role of Antisense Technology in Target Validation
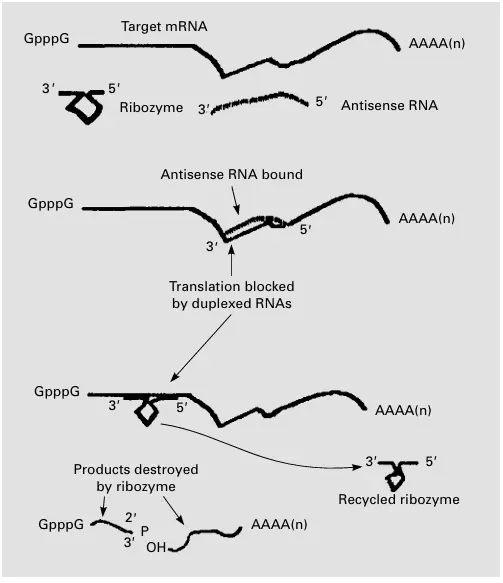
One of the most advanced and effective tools for validating therapeutic targets is antisense oligonucleotides (ASOs). These are short, synthetic strands of nucleotides designed to bind to specific mRNA molecules, effectively "silencing" the gene expression associated with a disease.
Antisense technology works by preventing the translation of the target mRNA into its corresponding protein. By "turning off" a gene using ASOs, researchers can observe the resulting changes in cell behavior, disease progression, or treatment response. This process is an excellent way to confirm whether a gene plays a critical role in disease pathology.
Antisense oligonucleotides are widely used in genetic research and drug discovery to study gene function, explore new therapeutic targets, and potentially treat genetic disorders by targeting the root cause.


GeneBlocs
GeneBlocs are small synthetic DNA/RNA hybrid molecules that bind to a specific target mRNA and induce its destruction by RNase H. GeneBlocs are constructed with a minimum content of antisense DNA to provide stability while ensuring they are non-toxic. Protective end groups and naturally occurring modifications to the RNA moieties provide further stability, allowing GeneBlocs to be effective over several days.
GeneBlocs behind the target mRNA with high affinity
GeneBlocs (23-mer) have also been designed to bind their target mRNA with high affinity and specificity so that nearly all (up to 98%) target mRNA is destroyed.
These macromolecules show good cellular uptake and are small enough to allow cost-effective manufacture.

GeneBlocs can be delivered with high efficiency
GeneBlocs need to be delivered into the cell where they exert their effects. atugen has developed more than 550 lipid formulations that are serum compatible and deliver GeneBlocs into a wide variety of cell lines. From these formulations, we can typically achieve over 95% tranfection efficiency for most cell lines. In addition, these lipid delivery vehicles promote the sustained delivery of GeneBlocs over several days. Evidence suggests that the lipid/GeneBloc vehicles accumulate at the cell surface and are taken into the cell by natural endocytotic processes. Pools of delivery vehicles on the cell surface and in lysosomes act as stores that slowly release GeneBlocs into the cell.

GeneBlocs are axtremely specific
GeneBlocs function by binding to any region of the target mRNA and thereby facilitating its destruction. It therefore follows that GeneBlocs do not need to bind to any particular mRNA region, such as that coding for the active site of the translated protein. GeneBlocs can therefore be designed to Knock-down an entire gene family or be targeted at specific family members.
Cell receptors can also be specifically targeted. For example, the VEGF receptor is known to promote angiogenesis (formation of new blood vessels). VEGF GeneBlocs inhibit VEGF-induced angiogenesis in vivo. The rat corneal model was used to show that GeneBlocs can be delivered locally and that their effect are sustained for several days following a single injection.

The biological effects of GeneBlocs are reversible
The effects of a GeneBloc are sustained over several days, however, after the first 5 days the effects gradually disappear, allowing normal translation of mRNA to resume and normal biological function to be restored. An example of this reversibility has been demonstrated in mice with estrogen-dependent tumors. These mice showed no tumor growth when they were treated with an estrogen receptor GeneBloc. After 15 days, treatment was stopped and tumor growth resumed at the same rate as control mice given a control GeneBloc and estrogen only.

GeneBlocs can be applied at different stages of development
Mouse embryos treated with a VEGF GeneBloc at a specific time during development demonstrate inhibited growth. VEGF is a mitogen known to be critical in regulating growth. Four spliced variants of the mitogen have been reported and transgenic VEGF knock-out mice, in which all variants are knocked-out, are not viable. Because this knock-out is lethal in embryos it therefore cannot be studied. Conditional knock-outs, in which 2 spliced variants are knocked-out, produce embryos that are viable and show severe cardiovascular defects. Experiments with these mice and their embryos are notoriously difficult to perform.
Using atugen’s technology, it is possible to design GeneBlocs that can knock-down any or all spliced variants and can be easily administered (i.p. or i.v.) at different stages of development.



Ribozymes: Cutting Genes at the Core
While antisense oligonucleotides and siRNA provide a way to silence genes, ribozymes offer a more direct approach. Ribozymes are catalytic RNA molecules that can cut specific RNA sequences, effectively "removing" the expression of a gene.
In target validation, ribozymes can be engineered to recognize and cleave the mRNA of a gene of interest. By removing the expression of the target gene, researchers can observe the effects on disease progression or cellular behavior. Ribozymes provide a unique, effective way to validate targets, particularly when working with genes that may be difficult to silence with traditional antisense or siRNA methods.





From In Vitro to In Vivo: Combining Tools for Better Results
While in vitro models (cell cultures) are essential for initial target validation, they have limitations. A gene that appears to be essential for disease progression in cultured cells may not behave the same way in an organism. To overcome this, researchers must confirm their findings using in vivo models, such as genetically modified mice, that more closely mimic human diseases.
By using both in vitro and in vivo systems, researchers can confirm that a target is truly essential for disease progression. The combination of gene silencing with siRNA, antisense technology, and ribozymes in both cell cultures and animal models is crucial for robust target validation. These tools allow scientists to observe the real-world effects of gene silencing and confirm whether modulating the target can lead to therapeutic benefits.